Vaccinating Over 65s: Lifesaving or a Misplaced Bet?
Vaccines are supposed to be the cornerstone of public health, especially to protect the elderly - but do they work well enough for older adults?
Much has been said and written about the Covid-19 ‘vaccines’, and World Council for Health has been calling for their withdrawal since November 2021. But what about vaccines in general? While many have concluded that all vaccines are best avoided by all groups, opinion is divided.
However, even if you take the position that vaccines are an essential pillar of public health policy - the majority position - there’s a problem. Because even within that paradigm, vaccines are failing certain groups they’re supposed to protect. In this article, WCH Science Lead Christof Plothe explains…
Aging and Immunity: A Fractured Defense System
Christof Plothe DO
The immune system doesn’t age gracefully. As we grow older, it becomes slower and less reliable - a process called immunosenescence - weakening the body’s ability to respond to infections or vaccines. This decline affects both the innate immune system (the first line of defense) and the adaptive immune system (which creates targeted antibodies and immune memory). The results? Vaccines, which rely on stimulating these systems, often fall short for older individuals. Here’s what happens in older adults after vaccination:
Lower antibody titers: the immune system produces fewer antibodies, meaning less protection.
Delayed response: it takes longer for the immune system to kick into gear.
Short-lived immunity: even when antibodies are produced, they fade more quickly than they do in younger people.
So with this in mind, are we putting too much stock in vaccines for older populations when their immune systems may not respond effectively? Let’s critically examine the science, the challenges, and the controversies.
Influenza vaccines: a waning shield
The flu vaccine is a public health staple, but recent studies have unveiled some eye-opening challenges regarding their effectiveness.
A 2023 study published in Immunity & Ageing revealed that, despite the development of vaccines specifically designed for older adults, the overall efficacy remains disappointingly low. This is largely due to age-related declines in immune response, which can leave our seniors more vulnerable to the virus.
The study pointed out that the effectiveness of the vaccine can vary significantly based on several factors, including the individual's age, overall health, and the virulence of the seasonal flu strain. This variability often results in lower antibody responses among older adults, compared to younger populations. A systematic review from 2024 confirmed that both young adults and the elderly experience lower vaccine effectiveness compared to other age groups. If the vaccines are least effective for the groups supposedly most ‘in need’ of them, what’s the point?
Moreover, a comprehensive analysis by Anderson et al. in the Annals of Internal Medicine examined data from a staggering 170 million episodes of care and 7.6 million deaths. They found NO evidence that influenza vaccination significantly reduced hospitalizations or mortality among the elderly. This challenges long-held assumptions about the benefits of vaccination for this vulnerable group.
A 2017 review by McElhaney et al. found that hemagglutination inhibition (HI) antibody responses—the standard measure of flu vaccine efficacy—don’t reliably persist year-round in older adults. Essentially, many older people lose their vaccine-induced immunity before flu season even peaks.
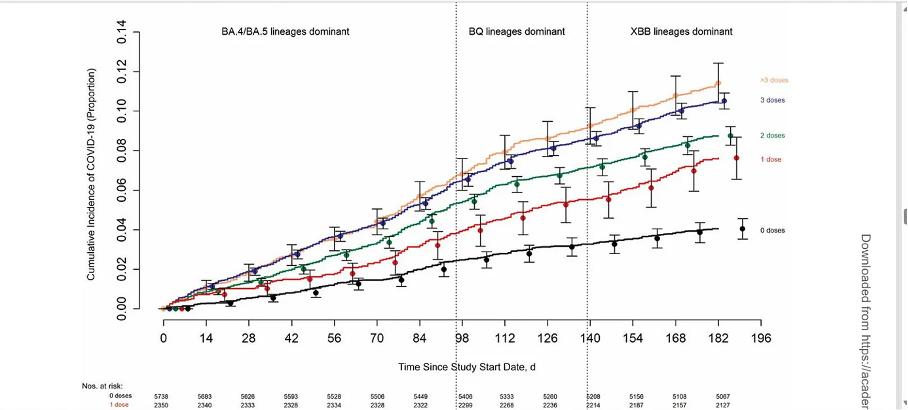
It’s not just that the effectiveness of these vaccines is in question - evidence suggests they actually make things worse. Have a look at this graph:
It shows that the higher the incidence of the influenza vaccine, the higher the COVID-19 death rate.
Pneumococcal vaccines: a leaky roof
The 23-valent pneumococcal polysaccharide vaccine (PPV23) is designed to protect against pneumonia and invasive pneumococcal disease. While widely used, it’s not particularly effective for older adults, offering at best 50% protection against pneumonia. Worse, antibody levels drop back to pre-vaccination levels within 6-10 years.
Covid-19 vaccines: a serious concern
Covid-19 ‘vaccines’ in their performance in older populations remains a concern. A study by Collier et al. (2021) revealed that individuals over 80 had significantly lower neutralizing antibody titers after receiving the Pfizer-BioNTech vaccine compared to younger participants. Alarmingly, many elderly participants failed to produce detectable antibodies even after their second dose. Another study noted that while the Moderna vaccine (mRNA-1273) generated slightly stronger responses than Pfizer-BioNTech (BNT162b2), overall antibody levels in older adults were still underwhelming. After several months, all mRNA-based “vaccines” showed a negative efficiency; people were more prone to get Covid-19 than the unvaccinated.
Why are older adults less responsive?
Blame it on biology. The aging immune system faces several hurdles that make vaccine responses weaker and less reliable:
Immunosenescence: Aging disrupts the balance between short-lived effector T cells and long-lived memory T cells, reducing the body’s ability to "remember" pathogens.
B-cell Defects: Aging B cells are less effective at producing antibodies—the very molecules vaccines are designed to generate.
Chronic Inflammation (Inflammaging): Older adults often experience persistent low-grade inflammation, which interferes with vaccine efficacy.
Underlying Health Conditions: Obesity, diabetes, and cardiovascular disease—all common in older adults—further impair immune function.
Is there a future of vaccination for the elderly?
The current "one-size-fits-all" approach to vaccination is clearly inadequate for older adults. Scientists are exploring innovative ways to boost vaccine responses in aging populations:
High-dose vaccines: Increasing the antigen dose is tested for influenza vaccines. For example, high-dose trivalent flu vaccines (HD-TIV) generate stronger immune responses in older adults. But do they actually create less symptoms or do they protect from severe outcomes? We don’t know.
Adjuvants: Adding immune-stimulating compounds like MF59 to vaccines can enhance antibody production and T-cell activation. Increasing the dosage of adjuvants pose many risks and no long term studies on the topic exist.
Booster doses: Regular booster shots, especially for COVID-19 vaccines, are supposed to help maintain antibody levels and extend immunity but lead to an antibody switch to IG G4 which make antibodies ineffective and people become more prone to have the infections and severe outcomes.
But more than that. A switch to IG G4 antibodies in the Covid injections not only makes the individual more likely to get Covid but can predispose the body to develop cancer. The same is known for flu injections. So is it a good idea to just repeat the injections as often as we can?
Tailored vaccines: Designing vaccines specifically for older adults, such as those targeting the unique aspects of immunosenescence, is supposed to be a new area of research. But no satisfying result has come out of this yet.
When it comes to vaccines for older adults, things aren't as straightforward as we'd like them to be. While vaccines aim to protect us, they aren't perfect for older folks and can sometimes lead to more infections or severe outcomes. In some cases, they might even switch to less effective antibodies (so-called IG G4s), which raises concerns, especially since the number of people over 65 is on the rise, making them more vulnerable to viral infections.
So, what’s the alternative?
Exploring a better way
In 2021, I presented evidence of health-supporting lifestyle changes in front of the Italian Senate. The proof of their role in tackling a viral outbreak (Covid-19) was convincing. These changes showed great results, with people asking for advice from all over the world with various risk factors for potentially severe outcomes, one of them being age. You can read my protocol in the guide below:
Ensuring that older adults have enough Vitamin D (ideally between 50-80 ng/ml) could dramatically reduce the risk of severe outcomes from illnesses like Covid-19—some studies suggest it could lead to close to zero deaths!
Additionally, cutting down on sugar during an infection might help, as sugar can worsen the disease in various ways (Logette, 2021). And why not also consider other micronutrients? Vitamin C, Zinc, and phytochemicals like Quercetin (a compound known to help zinc enter cells) could be key players in preventing viral infections.
Zinc has shown antiviral effects for years and specifically targets the machinery viruses use to replicate. Interestingly, there are substances called zinc ionophores that help zinc get into cells more effectively, blocking viral entry. Besides chloroquine, quercetin and epigallocatechin-gallate (found in green tea) are also zinc ionophores. In fact, combining quercetin with zinc is currently being tested in clinical trials for treating Covid-19 and other viral infections.
So, why is the focus for protecting older adults from infections almost solely on vaccines? There are other options, like repurposed medications and micronutrients, that could potentially be more effective in preventing severe outcomes. The World Council for Health advocates for exploring these alternatives to find a better approach to keeping older adults healthy and safe.
References:
1. Anderson ML, Dobkin C, Gorry D. The effect of influenza vaccination for the elderly on hospitalization and mortality: an observational study with a regression discontinuity design. Ann Intern Med. 2020 Apr 7;172(7):445-52. https://pubmed.ncbi.nlm.nih.gov/32120383/.
2. Anderson, M. L., Dobkin, C., & Gorry, D. (2020). The Effect of Influenza Vaccination for the Elderly on Hospitalization and Mortality: An Observational Study With a Regression Discontinuity Design. Annals of Internal Medicine.
3. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017 Jul;16(7):723,733. https://pubmed.ncbi.nlm.nih.gov/28562111/.
4. Borsche L, Glauner B, von Mendel J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng/mL 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients. 2021 Oct 14;13(10):3596. doi: 10.3390/nu13103596. PMID: 34684596; PMCID: PMC8541492.
5. Cadar AN, Martin DE, Bartley JM. Targeting the hallmarks of aging to improve influenza vaccine responses in older adults. Immun Ageing. 2023 May 17;20(1):23. doi: 10.1186/s12979-023-00348-6. PMID: 37198683; PMCID: PMC10189223.
6. Canaday, D. H., et al. (2022). Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naive nursing home residents. Clinical Infectious Diseases, 75(1), e708-e711.
7. Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Selecting viruses for the seasonal influenza vaccine; [cited 2020 Aug 17]. https://www.cdc.gov/flu/prevent/vaccine-selection.htm.
8. Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC seasonal flu vaccine effectiveness studies; [cited 2020 Apr 17]. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm.
9. Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. How the flu virus can change: ‘drift’ and ‘shift’; [cited 2020 Aug 17]. https://www.cdc.gov/flu/about/viruses/change.htm.
10. Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. How flu vaccine effectiveness and efficacy are measured; [cited 2020 May 14]. https://www.cdc.gov/flu/vaccines-work/effectivenessqa.htm.
11. Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about underlying cause of death, 1999-2018; [cited 2020 May 2]. https://wonder.cdc.gov/ucd-icd10.html; query for death from influenza, 2000-2003. Between 2000 and 2003, there were 61 annual deaths from influenza out of 77 million children age 18 and younger, about 1 death in 1.26 million.
12. Ciabattini, A., et al. (2018). Vaccination in the elderly: The challenge of immune changes with aging. Seminars in Immunology, 40, 83-94.
13. Ciabattini, A., et al. (2021). Vaccination in the elderly: The challenge of immune changes with aging. Seminars in Immunology, 51, 101525.
14. Collier, D. A., et al. (2021). Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature, 596(7872), 417-422.
15. Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, Ortiz M, O'Sullivan CK, Fernández-Larrea JB. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1-6 cells to a liposome model. J Agric Food Chem. 2014 Aug 13;62(32):8085-93
16. Del Giudice, G., et al. (2018). Fighting against a protean enemy: immunosenescence, vaccines, and healthy aging. NPJ Aging and Mechanisms of Disease, 4, 1.
17. Demicheli V, Jefferson T, Al-Ansary LA, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database of Syst Rev. 2014 Mar 13;(3):CD001269. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001269.pub5/epdf/full.
18. Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001269.pub6/full.
19. Demicheli, V., et al. (2020). Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews, 2(2), CD004876.
20. DiazGranados, C. A., et al. (2014). Efficacy of high-dose versus standard-dose influenza vaccine in older adults. New England Journal of Medicine, 371(7), 635-645.
21. Dierig A, Heron LG, Lambert SB, Yin JK, Leask J, Chow MY, Sloots TP, Nissen MD, Ridda I, Booy R. Epidemiology of respiratory viral infections in children enrolled in a study of influenza vaccine effectiveness. Influenza Other Respir Viruses. 2014 May;8(3):293-301. Epub 2014 Jan 31. https://pubmed.ncbi.nlm.nih.gov/24483149/.
22. Falkenhorst, G., et al. (2017). Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLoS One, 12(1), e0169368.
23. Falsey, A. R., et al. (2021). SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. New England Journal of Medicine, 385(17), 1627-1629.
24. Guo J, Chen X, Guo Y, Liu M, Li P, Tao Y, Liu Z, Yang Z, Zhan S, Sun F. Real-world effectiveness of seasonal influenza vaccination and age as effect modifier: A systematic review, meta-analysis and meta-regression of test-negative design studies. Vaccine. 2024 Mar 19;42(8):1883-1891. doi: 10.1016/j.vaccine.2024.02.059. Epub 2024 Feb 28. PMID: 38423813.
25. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB; Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2004 May 28;53(RR-6):1-40. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm.
26. Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Sys Rev. 2010 Jul 7;(7):CD001269. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001269.pub4/full.
27. Jefferson T. Influenza vaccination: policy versus evidence. BMJ. 2006 Oct 28;333(7574):912-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1626345/.
28. Joshi AY, Iyer VN, Hartz MF, Patel AM, Li JT. Effectiveness of trivalent inactivated influenza vaccine in influenza-related hospitalization in children: a case-control study. Allergy Asthma Proc. 2012 Mar-Apr;33(2):e23-7. https://pubmed.ncbi.nlm.nih.gov/22525386/.
29. Liu, Q., et al. (2022). Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. The Lancet, 398(10303), 856-869.
30. Logette E, Lorin C, Favreau C,. Oshurko E, Coggan JS, Casalegno F,. Sy MF, Monney C, Bertschy M,. Delattre E, Fonta P-A, Krepl J,; A Machine-Generated View of the Role of Blood Glucose Levels in the Severity of COVID-19. HYPOTHESIS AND THEORY published: 28 July 2021, doi: 10.3389/fpubh.2021.695139
31. McElhaney, J. E., et al. (2010). T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Frontiers in Immunology, 1, 41.
32. Nikolich-Žugich, J., et al. (2022). The twilight of immunity: emerging concepts in aging of the immune system. Nature Immunology, 23(2), 182-191.
33. Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013 May;56(10):1363.
34. Pera, A., et al. (2015). Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas, 82(1), 50-55.
35. Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Vaccines: what about immunocompromised schoolchildren? Dec 2019. https://physiciansforinformedconsent.org/immunocompromised-schoolchildren/rgis/.
36. Prasad, S., et al. (2022). Effectiveness of Common and Experimental COVID-19 Vaccines Against SARS-CoV-2 Delta Variant: A Systematic Review and Meta-Analysis. Vaccines, 10(3), 350.
37. Raszek M, Cowley D, Redwan E, Uversky V, Rubio-Casillas A, Exploring the possible link between the spike protein immunoglobulin G4 antibodies and cancer progression, Explor Immunol. 2024;4:267–284 DOI: https://doi.org/10.37349/ei.2024.00140
38. Read SA, Obeid S, Ahlenstiel C, et al. The role of zinc in antiviral immunity. Adv Nutr2019;10:696-710.
39. Rikin S, Jia H, Vargas CY, Castellanos de Belliard Y, Reed C, LaRussa P, Larson EL, Saiman L, Stockwell MS. Assessment of temporally related acute respiratory illness following influenza vaccination. Vaccine. 2018 Apr 5;36(15):1958-64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7115556/.
40. Sablerolles, R. S., et al. (2022). Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming. New England Journal of Medicine, 386(10), 951-963.
41. Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005 Feb 14;165(3):265-72. https://pubmed.ncbi.nlm.nih.gov/15710788/.
42. Soiza, R. L., et al. (2021). Efficacy and safety of COVID-19 vaccines in older people. Age and Ageing, 50(2), 279-283.
43. Steenhuis, M., et al. (2021). Specific antibody responses against SARS-CoV-2 variants after vaccinaton. Frontiers in Immunology, 12, 687821.
44. Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database Syst Rev. 2016 Jun 2;(6):CD005187. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005187.pub5/full.
45. Wilkinson, K., et al. (2017). Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine, 35(21), 2775-2780.
46. Xu, S., et al. (2022). COVID-19 Vaccination and Non–COVID-19 Mortality Risk — Seven Integrated Health Care Organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb Mortal Wkly Rep, 71(42), 1319-1325.
47. Yau, K., et al. (2022). The use of COVID-19 vaccines in children and adolescents. The Lancet Infectious Diseases, 22(1), e17-e18.
48. Zimmermann, P., et al. (2023). Immunogenicity and safety of pneumococcal conjugate vaccines in adults: A systematic review and network meta-analysis. The Lancet Infectious Diseases, 23(1), 128-140.






ALL VACCINES MAIME AND KILL.
The answer to that last question is, of course, insatiable greed. If they aim to vaccinate everybody they could surely use their scientists' ingenuity to tailor the vax to different age ranges, including kids - many of us would still refuse,of course, and they won't in any case, because of their insatiable greed and contempt for humans.