RSV ‘vaccines’ revealed
Does the ‘danger’ of the disease justify applying untested technologies on the most vulnerable: pregnant mothers, newborns, and the elderly ?
Countless concerned parents have asked the WCH about the new RSV vaccines. In this post, we look at what they are, as well as the virus itself, with the aim of helping readers make their own informed decisions. The post is in two parts: first, a summary of the most salient points. Then, a more academic paper into the context and science for health professionals and others looking to gain a deeper perspective.
Part 1: The ‘TLDR’ summary
RSV symptoms are mild and mimic the common cold. Most babies have been infected with RSV by their second birthday. In the EU, more than 90% of hospitalized adult RSV patients are over 65 years old.
It is easily treated with nebulizer therapy. Urgent care and hospitalization can occur for serious cases and if treated early, infant mortality should not be a concern. Among the 22.4 million children under 5 years old in the US, the annual risk of RSV hospitalization is well under 1%.
RSV ‘vaccines’ only reduce the risk of hospitalization from RSV by 1%.
So-called RSV ‘vaccines’ fall into three categories: monoclonal antibodies, a protein-based ‘vaccine’, and mRNA technology.
The monoclonal antibody treatment is called nirsevimab and is given in a single dose. There are serious safety concerns around nirsevimab. The clinical trials had limitations and there is little to no long-term safety data. Ambiguity around its classification also complicates safety monitoring and accountability.
Some reports link nirsevimab to infant deaths. Many treated infants still end up in hospital, and resistant strains of the virus are emerging. Antibody-dependent enhancement (ADE) is also a concern.
Recent vaccines developed by GSK and Pfizer for pregnant women have shown a 2% increase in premature births and higher rates of neonatal deaths in trials.
Moderna’s mResvia mRNA vaccine is recommended by the European Medicines Agency for the over sixties, yet with no data showing it’s either safe or effective. The same safety concerns exist for mResvia as for any other mRNA vaccine, namely myocarditis, auto-immunity, genomic integration and cancer.
There are alternatives. Studies show a clear inverse relationship of severity of RSV symptoms and Vitamin D levels. Better vitamin D levels may lower the incidence of RSV-associated bronchiolitis in infants, and vitamin D helps enhance immune response, reduce inflammation and helps stop RSV getting into cells. Quercetin and zinc are also worth consideration as part of a treatment protocol.
If you’d like to discuss these points with your doctor or other health professional, consider sharing the following detailed paper with them. It includes aspects many vaccinating doctors have not been informed about so please discuss it with them before potential injections.
Part 2: A deep-dive into RSV and the novel injections now recommended as treatment
Introduction: where did RSV even come from, anyway?
In the mid-1950s, research was underway to mass-produce the polio vaccine, which involved growing viruses in monkey kidney cells, leading to the shipment of hundreds of thousands of monkeys to the U.S. In late 1955, a troop of chimpanzees at the Walter Reed Army Institute developed respiratory illness, and researchers isolated the causative agent, naming it Chimpanzee Coryza Agent Virus (CCA). This virus was later linked to a respiratory infection in a human worker, prompting a name change to Respiratory Syncytial Virus (RSV), which became the preferred term in medical literature.

Further studies showed that inoculating susceptible chimpanzees with CCA resulted in illness, and by 1957, researchers identified a virus related to CCA in infants with respiratory illnesses. This virus was found in children suffering from pneumonia and bronchiolitis in the Maryland-District of Columbia area. By 1961, additional specimens resembling CCA were isolated. Before 1960, influenza and parainfluenza viruses were the primary causes of respiratory infections in infants. However, by July 1961, there was a significant increase in cases of bronchiolitis and bronchitis, particularly among infants under 12 months. Research suggested that the initial chimpanzee virus likely originated from a human infection (Morris, 1965; Chanock, 1957).
In 2005, the mainstream German paper Die Welt reported that doctors suspected a connection between the introduction of the measles vaccination (in Germany since 1973), which a large proportion of mothers were given at the time, and the increased susceptibility of their children to the RS virus. This assumption was supported by the fact that both the measles virus and the RS virus belong to the same family of paramyxoviruses. It also appears, the doctors write, that in countries with low measles vaccination rates, susceptibility to severe childhood respiratory infections requiring hospitalisation is lower (Welt, 2005).
Within five years of the virus's discovery, hospitalizations for RSV-related illnesses surpassed those for influenza in children.
In adults and older healthy children, RSV symptoms are mild, mimicking the common cold. By the age of two, 97% of babies have been infected with RSV. In a Substack post, Dr. Meryl Nass, a physician and researcher, cited CDC data to state that 17 babies up to the age of one year died from RSV in the United States annually on average over a 12-year period. In the EU, more than 90% of hospitalized adult RSV patients are over 65 years old.
Dr Peter McCullough has reported that the respiratory syncytial virus (RSV) is a common viral infection affecting infants (~3.6 million) mainly under age 1 year, easily treated with nebulizer therapy. Urgent care, emergency room, and hospitalization can occur for serious cases and if treated early, infant mortality should not be a concern. Among the 22.4 million children under 5 years old, the annual risk of RSV hospitalization is well under 1%.
Studies show a clear inverse relationship of severity of RSV symptoms and Vitamin D levels. So why this urgency to distribute vaccines to an entire new generation?
New RSV vaccination in newborns and pregnant women
To combat RSV, ‘health experts’ worldwide now recommend a so-called preventive treatment for all newborns and infants. However, this treatment (‘vaccines’) only reduces the risk of hospitalization from RSV by a mere 1%.
The prophylactic measure involves giving a single dose of monoclonal antibody called nirsevimab, marketed as Beyfortus®. The history of RSV vaccines spans sixty years, marked by failures and safety concerns. Recent vaccines developed by GSK and Pfizer for pregnant women have shown a 2% increase in premature births and higher rates of neonatal deaths in trials.
Despite GSK withdrawing its vaccine due to safety worries, Pfizer pursued approval, claiming no significant safety issues. If widely administered, the vaccine could cause around 73,285 additional preterm births, potentially leading to more infant deaths than the lives saved from RSV hospitalizations, which raises serious ethical concerns about its approval and recommendation by health authorities. As of August 30, 2024, the CDC recommends: “Vaccination for pregnant people, 1 dose of maternal RSV vaccine during weeks 32 through 36 of pregnancy, administered immediately before or during RSV season.”
Moderna just received approval for an mRNA RSV gene therapy injection (“vaccine”). Here, as well, in the approval trial, we find an absolute risk reduction of <1%.
The European Medicines Agency (EMA) also approved this year the first vaccines against RSV: Arexvy from GlaxoSmithKline (GSK) for people aged 60 and over and Abrysvo from Pfizer for pregnant women, to provide infants with “artificial nest protection” through passive immunization on a protein basis.
New vaccine technology: monoclonal antibodies
Monoclonal antibodies are lab-made molecules designed to mimic our immune system's ability to fight off harmful invaders like viruses. They work by attaching to specific parts of the virus, helping the immune system recognize and attack it. Nirsevimab works by binding to the RSV fusion protein, which blocks the protein in its pre-fusion shape. This action prevents free virus particles from entering cells and stops the spread of the virus through cell fusion. When injected, the antibodies are immediately available to defend against RSV, having been genetically engineered from Chinese hamster ovary cells. According to the German CDC (STIKO), the protection lasts for “at least 6 months,” covering an RSV season.
Concerns and controversies
Despite the promise of this new treatment, there are several concerns. Some reports have linked nirsevimab to infant deaths, raising questions about its safety, especially since long-term safety data is limited. The effectiveness of this treatment is under scrutiny, as the absolute risk reduction of requiring hospitalization for an RSV infection is just 1%. The cost-effectiveness of administering nirsevimab to all infants is also being debated, particularly as many vaccinated infants still end up in the hospital, and some resistant strains of the virus are emerging (Beach, 2022). There are concerns about antibody-dependent enhancement (ADE), where antibodies may enhance viral infection. This risk could increase as antibody levels and wane over time.
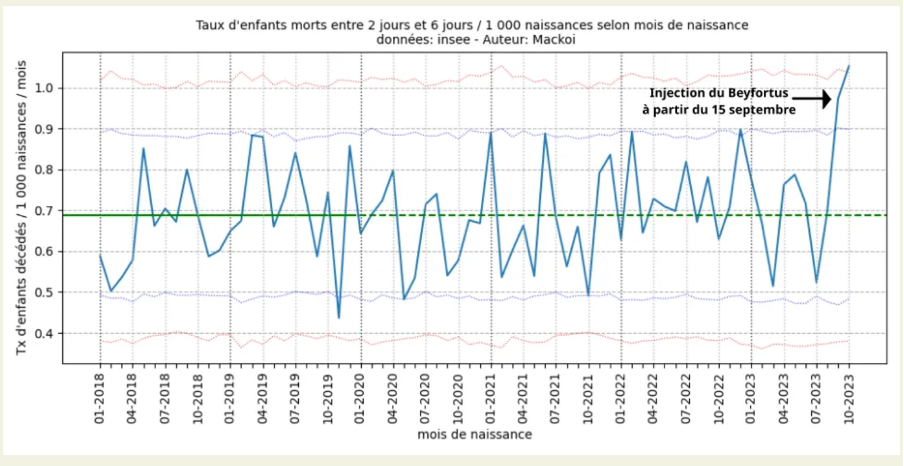
The blue curve here represents, for each month (from 2018 to October 2023), the mortality rates of 2 to 6 days of life, of the native babies of the month in question in France.
As of September 2023, there are 54 infant deaths between 2 and 6 days of life out of 55,489 births, a mortality rate of 0.97 deaths per 1,000 births. France was the world's testing ground for nirsevimab (Beyfortus). It was injected into newborns in maternity wards since September 2023 and led to a 50% increase in infant deaths between 2 and 6 days of age. The main adverse effect was bronchiolitis possibly by facilitation of infection by monoclonal antibody.
The Defender reported in March 2024 that it had been less than a year since the Centers for Disease Control and Prevention (CDC) recommended two new respiratory syncytial virus (RSV) vaccines — yet CDC data and the Vaccine Adverse Event Reporting System (VAERS) already showed reports of 34 deaths, 302 serious adverse events and according to reports at that time, a safety signal for Guillain-Barré syndrome (GBS).
Efficiency of the trials
The clinical trials for nirsevimab had significant limitations, making it difficult to fully assess its safety. Many trials were conducted during periods of low RSV circulation, meaning there weren't many cases of severe RSV to compare between treated and placebo groups. Some concerning trends emerged, such as babies who received nirsevimab and were still hospitalized for RSV staying longer in the hospital than those who received a placebo. Although the FDA noted that there were 12 deaths among 3,710 infants who received nirsevimab (0.32%) compared to four deaths among 1,797 infants in the control group (0.22%), these numbers raise concerns that deserve further scrutiny.
A significant number of participants were also excluded from final analyses in these trials—ranging from 2% to 8% of treated infants. This type of exclusion can mask safety signals or artificially inflate efficacy estimates. This treatment has not been tested on newborns, but on children aged between 3 months and 2 years. In its September 2022 report, the EMA reminds us of the fiasco of RSV vaccine trials in the past: children died of severe bronchiolitis in the vaccinated groups and none in the control groups (Banoun, 2024).
Classification by regulatory boards and considerations around liability
Nirsevimab occupies a unique position that straddles the classifications of both drugs and vaccines. The CDC interchange these definitions as it suits their needs. By categorizing nirsevimab as a vaccine, manufacturers can secure liability protection by including it in the childhood vaccine schedule. However, for reimbursement purposes, it is classified as a drug. This dual classification also impacts how adverse events are reported. When nirsevimab is administered alone, any adverse event reports are directed to the FDA's drug reporting system (FAERS). Conversely, if it is given alongside other vaccines, reports are submitted to the vaccine reporting system (VAERS). This regulatory ambiguity complicates safety monitoring and accountability further (Banoun, 2024).
The new RSV vaccines for the elderly
Arexvy by Pfizer is the first FDA-approved vaccine designed to prevent respiratory syncytial virus (RSV) infections in older adults. The technology behind Arexvy involves recombinant DNA technology (recombinant glycoprotein F that stabilized in the pre-fusion conformation), which is crucial for eliciting an immune response against the virus. This glycoprotein is produced in Chinese Hamster Ovary (CHO) cells.

The mResvia vaccine (Moderna) has received a positive recommendation from the CHMP (the European Medicines Agency's committee) for use in adults aged 60 and older (Wilson, 2023). This vaccine is supposed to help to prevent serious respiratory illnesses caused by RSV, a virus that typically causes mild cold-like symptoms but can be dangerous for older people. Importantly, this is the first mRNA vaccine aimed at a virus other than SARS-CoV-2 (the virus that causes COVID-19) to get a positive recommendation from the CHMP. The conditional approval is granted without the EMA publishing any data to show that mResvia is not only effective but, above all, harmless. A study (Barmada, 2023) already demonstrated a worst-case scenario for any manufacturer of modRNA technology. According to the authors, it is not primarily the spike protein (like the Covid gene injections) that is toxic, but the transport platform, consisting of lipid nanoparticles and adjuvants, excipients designed to ensure that the modRNA of the spike protein enters cells and that the spike protein can be built there.
Dr McCullough has pointed out that the following safety concerns already exist for any modified (pseudouridinated), synthetic mRNA product including mResvia: It can cause myocarditis because mRNA of all types targets the heart (Krauson, 2023), auto-immunity because of the generation of foreign RSV proteins and frameshifted peptides (Boros, 2022), genomic integration (Alden, 2022) and oncogenicity (Cancer) (Seneff, 2022).
The role of Vitamin D and other substances
Research has shown that low levels of vitamin D, specifically 25-hydroxyvitamin D (25(OH)D), are linked to an increased risk of RSV infection, particularly in infants. Better vitamin D status may thus lower the incidence of RSV-associated bronchiolitis in infants (Maxwell, 2012). Additionally, vitamin D plays a crucial role in enhancing immune responses. It helps reduce inflammation and promotes the production of antiviral peptides that can block the entry of RSV into cells and prevent cell death.
Quercetin and Zinc are also worth mentioning. Quercetin, which is found in foods such as onions and apples, serves as a zinc ionophore. This means it helps facilitate the entry of zinc into cells, where it can inhibit viral replication. Furthermore, quercetin has demonstrated potential antiviral properties by blocking viral replication, especially when combined with zinc, thereby enhancing the overall immune response.
Conclusion
The controversies surrounding prophylaxis against RSV infections highlight the need for further research before launching a new technology on infants. Public health policy must be more balanced. The potential role of vitamin D and other substances in preventing RSV infections should be explored further, and the cost-effectiveness and safety of current prophylaxis methods should be critically evaluated. Finally, there will never be a vaccine for respiratory viruses to prevent infection or spread. That’s because vaccines work by prompting the body to produce antibodies - yet the body will only do this once the virus has entered the system in the blood. Respiratory viruses like RSV replicate in the nasal mucosa and thus will be spread from there in any case of infection. This renders any such vaccine at best, pointless, and at worst, extremely harmful.
Sources:
1. Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115-1126. https://doi.org/10.3390/cimb44030073
2. Banoun, H. Independent Analysis of the Results of the First Infant Immunization Campaign with Beyfortus® (Nirsevimab, Monoclonal Antibody against RSV, Bronchiolitis Virus): Mixed Results, Identification of Biases and Possible Role and Mechanisms of ADE (Antibody Dependent Enhancement). Preprints 2024, 2024060714. https://doi.org/10.20944/preprints202406.0714.v1
3. Barmada, Anis, Jon Klein, Anjali Ramaswamy, Nina N. Brodsky, Jillian R. Jaycox, Hassan Sheikha, Kate M. Jones et al. (2023). Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine–associated myocarditis. Science Immunology 8(83): eadh3455.“
4. Beach SS, Hull MA, Ytreberg FM, Patel JS, Miura TA. Molecular Modeling Predicts Novel Antibody Escape Mutations in the Respiratory Syncytial Virus Fusion Glycoprotein. J Virol. 2022 Jul 13;96(13):e0035322. doi: 10.1128/jvi.00353-22. Epub 2022 Jun 9. PMID: 35678603; PMCID: PMC9278155.
5. Boros LG, Kyriakopoulos AM, Brogna C, Piscopo M, McCullough PA, Seneff S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol Res Perspect. 2024 Jun;12(3):e1218. doi: 10.1002/prp2.1218. PMID: 38867495; PMCID: PMC11169277.
6. Chanock, Robert, Bernard Roizman, and Ruth Myers. "Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA): isolation, properties and characterization." American Journal of Epidemiology, Volume 66, Issue 3, November 1957, Pages 281–290
7. Defender 2024, https://childrenshealthdefense.org/defender/rsv-vaccines-deaths-serious-injuries/
8. https://gilbertlab.com/neutraceuticals/quercetin/antiviral-effects-of-quercetin-zinc-ionophore/
9. https://journals.sagepub.com/doi/abs/10.3181/00379727-92-22538
10.
11. https://sashalatypova.substack.com/p/protect-babies-from-the-injection?utm_source=post-email-title&publication_id=870364&post_id=139679968&utm_campaign=email-post-title&isFreemail=true&r=kzd9f&triedRedirect=true
12. https://welcometheeagle.substack.com/p/bombshell-medical-cartel-is-killing?utm_source=post-email-title&publication_id=1514673&post_id=142310678&utm_campaign=email-post-title&isFreemail=true&r=kzd9f&triedRedirect=true&utm_medium=email
13. https://www.cdc.gov/rsv/index.html
14. https://www.ema.europa.eu/en/medicines/human/EPAR/arexvy
15. https://www.healthfirstdc.com/blog/the-importance-of-zinc-and-quercetin-during-pandemic
16. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3360794/
17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC353050/
18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7551809/
19. Krauson AJ, Casimero FVC, Siddiquee Z, Stone JR. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines. 2023 Sep 27;8(1):141. doi: 10.1038/s41541-023-00742-7. PMID: 37758751; PMCID: PMC10533894.
20. Maxwell CS, Carbone ET, Wood RJ. Better newborn vitamin D status lowers RSV-associated bronchiolitis in infants. Nutr Rev. 2012 Sep;70(9):548-52. doi: 10.1111/j.1753-4887.2012.00517.x. Epub 2012 Aug 17. PMID: 22946854.
21. Morris, J. A., R. E. Blount Jr, and R. E. Savage. "Recovery of cytopathogenic agent from chimpanzees with coryza." Proceedings of the Society for Experimental Biology and Medicine 92.3 (1956): 544-549
22. Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022 Jun;164:113008. doi: 10.1016/j.fct.2022.113008. Epub 2022 Apr 15. PMID: 35436552; PMCID: PMC9012513.
23. Welt, 2005: https://www.welt.de/print-welt/article177842/Spaetwirkung-nach-Masernimpfung.html
24. Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, Monroy J, Duncan CJA, Ujiie M, Rämet M, Pérez-Breva L, Falsey AR, Walsh EE, Dhar R, Wilson L, Du J, Ghaswalla P, Kapoor A, Lan L, Mehta S, Mithani R, Panozzo CA, Simorellis AK, Kuter BJ, Schödel F, Huang W, Reuter C, Slobod K, Stoszek SK, Shaw CA, Miller JM, Das R, Chen GL; ConquerRSV Study Group. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N Engl J Med. 2023 Dec 14;389(24):2233-2244. doi: 10.1056/NEJMoa2307079. PMID: 38091530.