‘A Very Dangerous Medical Experiment’: CDC Expands Vaccine Schedules for Kids, Pregnant Women and Most Adults
‘A Very Dangerous Medical Experiment’: CDC Expands Vaccine Schedules for Kids, Pregnant Women and Most Adults
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website.
The Centers for Disease Control and Prevention (CDC) is recommending more vaccines and vaccine doses across the board for children, pregnant women and adults, according to the agency’s 2024 immunization schedule.
The updated schedule triggered a flurry of news and reactions in recent days. However, the CDC released the updated schedule in September — months earlier than usual, to speed up insurance payments for newly recommended vaccines, the American Academy of Pediatrics reported.
The 2024 schedules include newly authorized recommendations for preventing COVID-19, respiratory syncytial virus (RSV), flu and pneumococcal disease.
“This amounts to nothing more than a very dangerous medical experiment foisted on America’s infants and children,” said Brian Hooker, Ph.D., senior director of science and research at Children’s Health Defense (CHD) and co-author of “Vax-Unvax: Let the Science Speak.”
Hooker told The Defender the CDC has never tested the efficacy or the safety of the entire childhood vaccination schedule.
Integrative physician Dr. Mary Kelly Sutton told The Defender, “The CDC continues to function as a powerful promoter of vaccines, not as a protector of public health.”
According to Sutton, who lost her license in three states for writing eight vaccine exemptions in California before the pandemic, there is little evidence of vaccines’ effectiveness and a lack of officially accepted research on their adverse effects. She said:
“The CDC could give wise guidance on vaccines but has so far accepted ACIP [Advisory Committee on Immunization Practices] approvals without studies. Universally, vaccines lack true placebo controls, and recently, lack human trials of any kind.
“The sad truth is pharma money rules the CDC, and the American people (and the world) are deceived and placed at risk.”
Commenting on the expanded vaccine schedule, Dr. Michelle Perro, a pediatrician and co-author of “What’s Making our Children Sick?” told The Defender, “It’s an outrage. It’s not necessary, and they’re hurting our children.”
Perro criticized the financial incentives offered to doctors to vaccinate as many people as possible, and the reprisals against doctors for writing exemptions.
Childhood (0-18) vaccine schedule
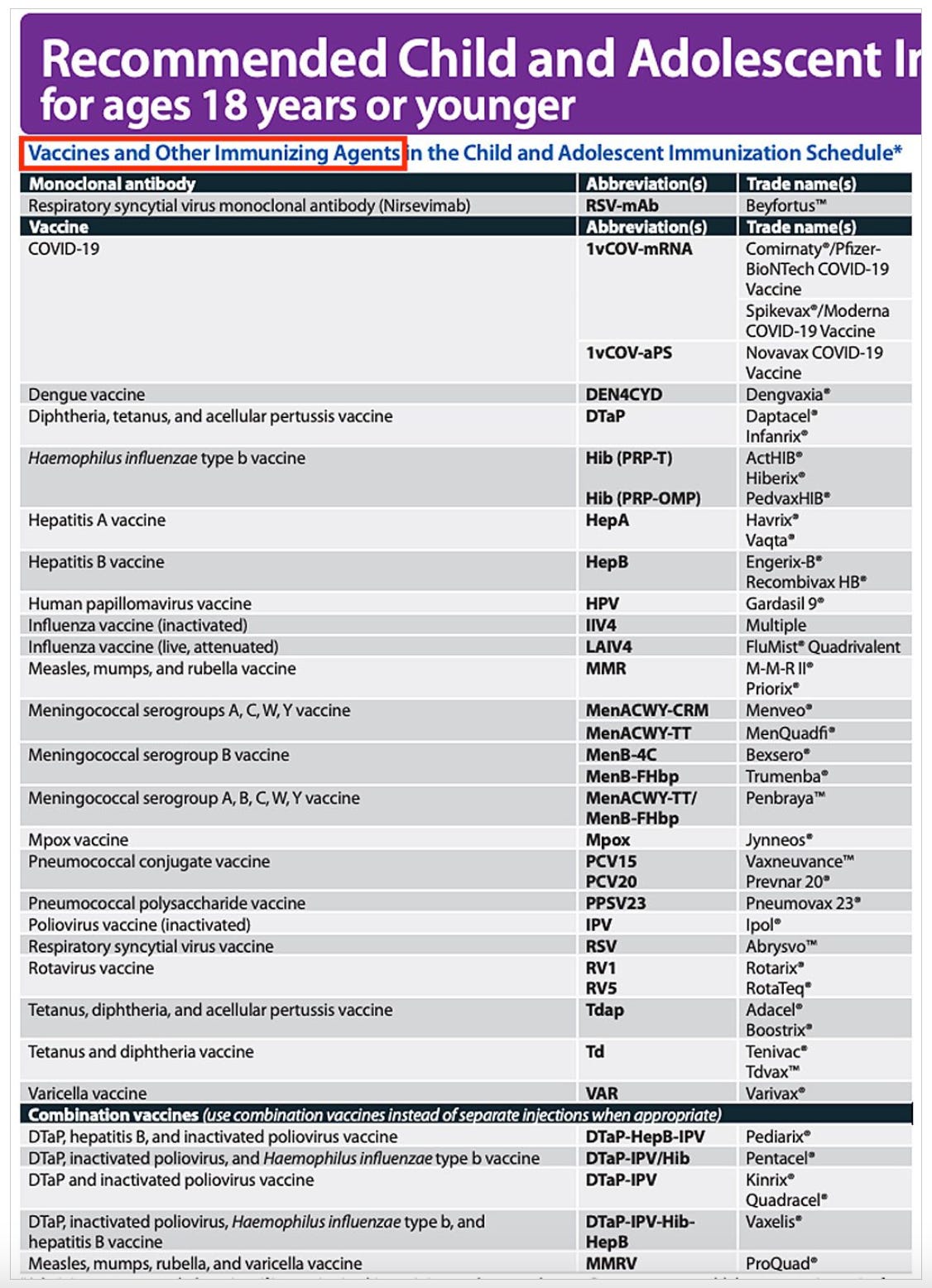
After carefully reviewing the new CDC schedules, CHD’s science team determined the likely minimum number for children ages 0-18 to be 76 doses of 18 different vaccines.
The number of doses could reach as high as 80, depending on the vaccine formulation being used and other factors.
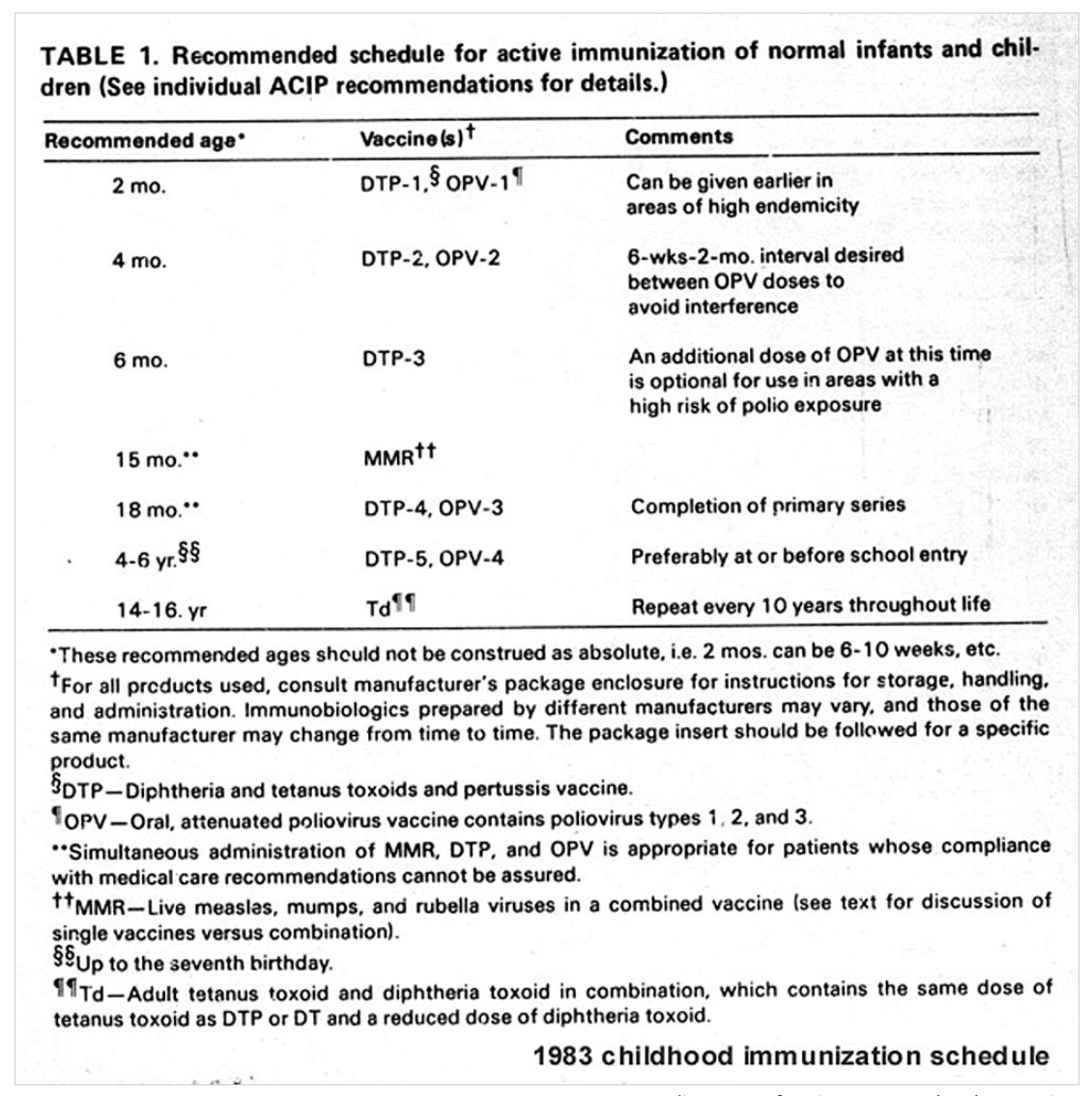
By comparison, the CDC in 1983 recommended 11 doses of 7 vaccines by age 16, including the MMR (measles, mumps, rubella), DTP (diptheria, tetanus, pertussis), and polio vaccines.
New immunizations on the childhood schedule include:
RSV: Nirsevimab (RSV-mAb, brand name Beyfortus) the monoclonal antibody treatment for children 0 through 8 months (if the mother did not receive the RSVpreF vaccine during pregnancy — see more information in the pregnancy section below), and certain high-risk children through 19 months. Additional guidance was added for locations with RSV seasonality that differs from that of the continental U.S.
Pneumococcal: a 20-valent pneumococcal conjugate vaccine (PCV20), targeting 20 strains of Streptococcus pneumoniae, in four doses: at 2 months, 4 months, 6 months, and 12-15 months. PCV20 replaces PCV13, the 13-valent pneumococcal conjugate vaccine.
COVID-19:
For the Moderna mRNA shots:
For children 6 months-4 years old: Those who have not been previously vaccinated are recommended to receive two doses of the “updated” 2023-24 Moderna vaccine. Those who have received one dose of any Moderna vaccine (including the updated version) or two doses of the older version are recommended to receive one dose of the updated vaccine.
For children 5 years and older: Those who have received zero, one or two doses of the older vaccine are recommended to get one dose of the updated Moderna vaccine for their age group. If they have been previously vaccinated with at least one dose of the updated vaccine, no further shots are recommended.
Note the timing for additional doses noted in the guidance.
Note the different recommendations on Table 2A for people who are moderately or severely immunocompromised.
For the Pfizer/BioNTech shots:
For children 6 months-4 years old: Those who have not been vaccinated are recommended to take the three-shot series of the updated Pfizer/BioNTech vaccine. Those who have received one dose of any version (old or new) of the vaccine are recommended to get two more shots to complete the series. Those who have received two doses of any version (old or new) are recommended to receive one more shot to complete the series. Those who have received three or more shots of the older vaccine are recommended to get one shot of the new version. For those who have received three or more shots, including at least one dose of the new formulation, no further shots are recommended.
For children 5 years and older: For those who are unvaccinated or have received any number of doses of the older Pfizer/BioNTech vaccine, they are recommended to get a single shot of the updated vaccine for their age group. For those who have previously received any number of doses including at least one of the new formulations, no further shots are required.
Note the timing for additional doses noted in the guidance.
Note the different recommendations on Table 2B for people who are moderately or severely immunocompromised.
Mpox (formerly monkeypox): Adults 18 and older who are at risk of mpox (gay, bisexual, transgender and nonbinary people with certain risk or exposure profiles) are recommended to receive the Jynneos vaccine. According to that guidance, the mpox vaccine appearing under the age-18 column on the childhood vaccine schedule is not expected to be administered to most children. Note that mpox clinical trials are currently underway for 12- to 17-year-olds.
Other changes to the childhood schedule include:
DTaP (diphtheria, tetanus, pertussis for 0-6 year-olds): The note on this vaccine was revised to clarify primary and booster doses.
Tdap (tetanus, diphtheria, and pertussis for 7-year-olds and older): The note was revised to clarify that the dose recommended for 11- to 12-year-olds is the adolescent booster dose.
HPV (human papillomavirus): The routine vaccination section includes clarification about doses not recommended for those who have already completed the HPV series.
Influenza: updated with recommended formulations for the 2023-24 flu season. Special notes about those with egg allergies have been removed; any person with a history of egg allergy can be vaccinated with these vaccines, according to the guidance.
MMR: The note was updated to specify use for routine, catch-up and “special situation” vaccinations.
MenB (meningococcal): Information about the newly licensed meningococcal A, B, C, W, Y vaccine has been added. A “shared clinical decision-making” document for individuals ages 16-23 was added.
Pneumococcal: Sections have been updated for routine, catch-up and “special situation” vaccinations, with new recommendations for the use of the 15-valent pneumococcal conjugate vaccine (PCV15) and PPSV23, in addition to the information about PCV20 noted above.
Poliovirus: New information has been added about catch-up vaccinations and increased risk exposure for 18-year-olds.
The CDC removed several vaccines from the schedule “because they no longer are distributed or recommended for use in the U.S.” These include bivalent mRNA COVID-19 vaccines, the diphtheria and tetanus toxoid vaccine (DT) and Menactra, a meningococcal vaccine.
The CDC published revised “vaccine catch-up guidance” for children who have fallen behind the recommended schedule. This includes guidance for the pneumococcal conjugate vaccine, Haemophilus influenzae type b vaccines (with guidance for different products), vaccines containing diphtheria, tetanus and pertussis (with specific guidance for formulations for different age groups) and the inactivated polio vaccine (IPV).
Additional catch-up guidance can be found in Table 2 of the childhood schedule.
The higher number of doses cited by attorney Aaron Siri in his tweet may have included the assumption that there would be yearly or biannual COVID-19 boosters, which the CDC schedule does not (yet) call for.
If annual boosters are added in the future, this could push the total number of doses through age 18 close to 100, if boosters begin at age 2.
‘Pregnant people’ vaccine schedule
The vaccine list for pregnant women includes four different shots: pertussis (whooping cough), flu (if pregnant during flu season), COVID-19 and RSV, with the latter administered between 32 and 36 weeks of pregnancy. Abrysvo’s inclusion on the childhood schedule is for pregnant adolescents only.
One dose of Pfizer’s bivalent RSVpreF vaccine Abrysvo is recommended for “pregnant people” to prevent RSV from later developing in their babies, despite concerns about premature births that stopped the development of a similar vaccine by GlaxoSmithKline (GSK).
There are currently no ACIP recommendations for RSV vaccinations in subsequent pregnancies.
The CDC recommends only one COVID-19 booster (2023-24 formulation) during pregnancy if the individual was previously vaccinated with the two-shot series pre-conception.
Adult (19 years and above) vaccine schedule
Adult recommendations include a minimum of 80 total doses of the following vaccines from ages 19-79, not including COVID-19 shots:
HepB (hepatitis B): Two, three or four doses, depending on vaccine or condition.
Tetanus, diphtheria, pertussis (Tdap or Td): One shot every 10 years.
Varicella (chicken pox): Two doses.
Influenza: 61 annual vaccines.
New vaccines include:
RSV: Either of two different vaccines for adults over 60 — GSK’s Arexvy or Pfizer’s Abryvso. Although the notes for this vaccine indicate it’s primarily for “those considered to be at increased risk for severe RSV disease” — which includes many chronic diseases such as COPD, diabetes mellitus, cardiovascular diseases, etc. — the CDC guidance includes recommendations for those who are frail, of advanced age or who reside in nursing homes or other long-term care facilities. Currently, a single dose is expected to provide prevention for two seasons. Concerning subsequent RSV doses, the CDC stated, “Additional surveillance and evaluation activities are planned to assess how long the vaccines protect against RSV and whether additional doses will be needed.”
COVID-19: Those previously unvaccinated are recommended to receive the updated 2023-24 formulations (of the mRNA vaccines, one- or two-dose series, depending on brand). Those previously vaccinated with one or more doses of any COVID-19 vaccine are recommended to take one dose of any updated (2023-24 formula) COVID-19 vaccine administered at least eight weeks after the most recent COVID-19 vaccine dose. Note the guidance for persons who are moderately or severely immunocompromised, as well as precautions and contraindications.
Other vaccines adults might receive, depending on medical conditions, exposure and risk factors, include zoster recombinant (RZV) (shingles), MMR, HPV, pneumococcal (PCV15, PCV20, PPSV23), hepatitis A, meningococcal A, C, W, Y, meningococcal B, Haemophilus influenzae type b and mpox.
The CDC lists under “shared clinical decision-making” recommendations for:
The HPV vaccine for adults ages 27-45 years old.
The MenB vaccine for individuals 16-23 years old.
The CDC updated its guidance around egg-based allergies and influenza vaccines, now saying that any person with a history of egg allergy can be vaccinated with any influenza vaccine (corresponding to age and health status) “with no additional safety considerations.”
All vaccine schedule changes can be found here.
One X user speculated about the role of immunizations and U.N. Agenda 2030, sharing a page from a World Health Organization (WHO) document titled “Immunization Agenda 2030”:
Nirsevimab not technically a vaccine
The National Vaccine Information Center wrote that nirsevimab (for children 0 through 19 months) is not technically a vaccine, but was added to the childhood schedule to give Sanofi and Astra Zeneca a liability shield and to get the monoclonal antibody treatment added to state mandates and vaccine registries.
Accordingly, the revised CDC schedule now states it is for “vaccines and other immunizing agents.”
Regarding liability, the CDC schedule states:
“The National Vaccine Injury Compensation Program (VICP) is a no-fault alternative to the traditional legal system for resolving vaccine injury claims. All vaccines included in the child and adolescent vaccine schedule are covered by VICP except dengue, PPSV23, RSV, Mpox and COVID-19 vaccines. Mpox and COVID-19 vaccines are covered by the Countermeasures Injury Compensation Program (CICP).”
To date, the CICP has compensated just 11 claims out of nearly 13,000 filed for COVID-19 vaccine injuries.
(In December, the Informed Consent Action Network, or ICAN, announced its support for a lawsuit against the U.S. Department of Health and Human Services to strike down the immunity protections and CICP provisions of the PREP (Public Readiness and Emergency Preparedness) Act, stating, “They violate the constitutional rights of those injured or killed by a COVID-19 vaccine.”)
Do vaccine combinations cause more harm?
Dr. Paul Thomas, author of “The Vaccine-Friendly Plan: Dr. Paul’s Safe and Effective Approach to Immunity and Health-from Pregnancy Through Your Child’s Teen Years,” commented on the lack of safety data on childhood vaccines, especially when combining vaccines.
“There is no study of the effects of the entire schedule,” he said. “As we have added more and more vaccines, we have ever-increasing chronic disease, neurodevelopmental issues, autoimmune disease and allergies.”
In their book, “Vax-Unvax,” Hooker and Robert F. Kennedy Jr., CHD’s chairman on leave, discussed studies indicating the potential harms of combining vaccines.
In one such study from 2012, comparing pregnant women receiving the flu shot, those receiving the shot in combination with the H1N1 vaccine were found to be 11 times more likely to have a miscarriage than those receiving the flu shot by itself.
The study’s author, Gary S. Goldman, an independent computer scientist, suggested that the increase in fetal loss may have been due to the additional dose of thimerosal in the H1N1 shot, exposing the fetus to mercury.
Goldman is also the co-author of a 2011 paper showing that developed countries requiring the most vaccine doses for infants had the least favorable infant mortality rates.
Goldman and co-author Neil Z. Miller, director of the Institute of Medical and Scientific Inquiry in Santa Fe, New Mexico, in a paper published in February 2023 replicated those findings and responded to critics.
In July 2023, they published a new study specifically examining the effects of two vaccines typically given to infants soon after birth — hepatitis B (HepB) and tuberculosis — from global data in 2019 and 2021. They found a strong link between vaccinations and rates of neonatal, infant and under-5 mortality for both years studied.
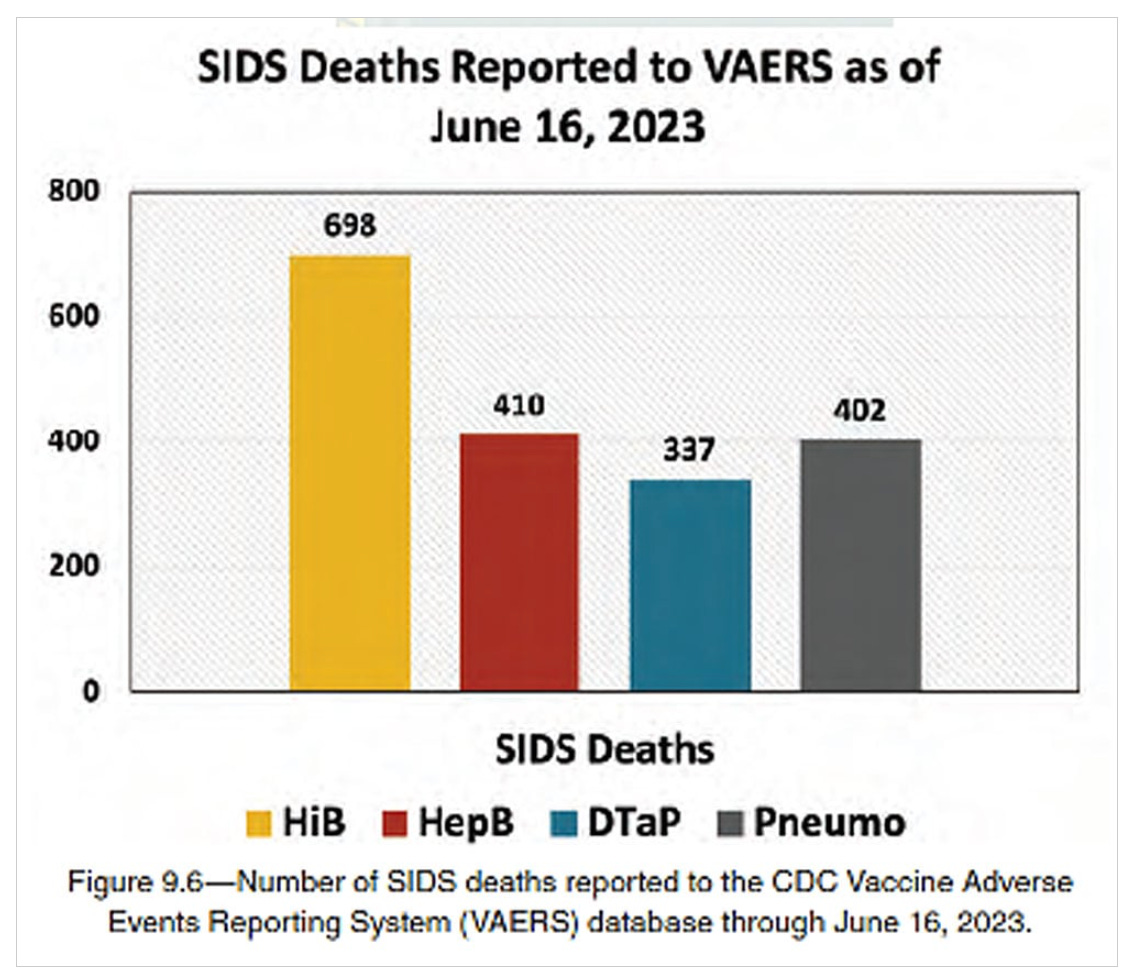
Another study included in “Vax-Unvax” reviewed the Vaccine Adverse Event Reporting System (VAERS) from 2005-2015 and found over 10,000 adverse events reports for infants receiving the HepB vaccine alone or with a multivalent vaccine, with 197 reports of sudden death syndrome (SIDS).
The chart below from “Vax-Unvax” shows the authors’ analysis of SIDS cases reported to VAERS after the hepatitis B, Haemophilus influenzae B, diphtheria-tetanus-acellular pertussis, and pneumonia vaccines.
ICAN last year raised concerns about combining the mpox, flu and COVID vaccines.
In March 2023 ICAN submitted a FOIA request to the CDC for evidence supporting claims made in its January 2023 tweet of a video from the White House MPox response Team stating, “You can get the #mpox vaccine at the same time as your #flu and #COVID vaccines.”
Dr. Demetre Daskalakis, then-acting director (now director) of the National Center for Immunization and Respiratory Disease, after stating in the video that the vaccines could be taken together, only offered that a person “may” consider delaying their COVID-19 shot for four weeks after getting the mpox vaccine due to a “possible risk” of heart inflammation.
ICAN stated the CDC video neglected to mention the connection between the COVID-19 vaccine and myocarditis and other heart injuries, which are also associated with the flu shot and mpox vaccine.
In its response to ICAN, the CDC failed to produce any documents supporting the safe co-administration of the three vaccines.
On Dec. 18, 2023, ICAN issued a statement titled, “CDC has no data to support its tweet telling America that it is safe to give the monkeypox, flu, and COVID shots together.”
This article was written by John-Michael Dumais and originally published by The Defender — Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.
Related content on WCH Substack:
If you find value in this Substack and have the means, please consider making a contribution to support the World Council for Health. Thank you.










On what basis did our medical moron industrial complex and their lackeys in white coats decide that vaccines work at all?
https://learntherisk.org/vaccines/diseases/
Horrific